What Happens to an Enzyme After a Reaction
Enzyme Active Site and Substrate Specificity
Enzymes catalyze chemic reactions past lowering activation energy barriers and converting substrate molecules to products.
Learning Objectives
Describe models of substrate binding to an enzyme's active site.
Key Takeaways
Key Points
- The enzyme 's agile site binds to the substrate.
- Increasing the temperature generally increases the charge per unit of a reaction, merely dramatic changes in temperature and pH can denature an enzyme, thereby abolishing its action equally a catalyst.
- The induced fit model states an substrate binds to an active site and both change shape slightly, creating an ideal fit for catalysis.
- When an enzyme binds its substrate information technology forms an enzyme-substrate complex.
- Enzymes promote chemical reactions by bringing substrates together in an optimal orientation, thus creating an ideal chemic surroundings for the reaction to occur.
- The enzyme will always return to its original land at the completion of the reaction.
Key Terms
- substrate: A reactant in a chemic reaction is called a substrate when acted upon by an enzyme.
- induced fit: Proposes that the initial interaction betwixt enzyme and substrate is relatively weak, merely that these weak interactions chop-chop induce conformational changes in the enzyme that strengthen bounden.
- active site: The active site is the part of an enzyme to which substrates bind and where a reaction is catalyzed.
Enzyme Active Site and Substrate Specificity
Enzymes bind with chemical reactants called substrates. There may be one or more substrates for each blazon of enzyme, depending on the particular chemic reaction. In some reactions, a unmarried-reactant substrate is broken downwardly into multiple products. In others, 2 substrates may come together to create one larger molecule. Two reactants might too enter a reaction, both go modified, and leave the reaction equally ii products.
The enzyme'due south active site binds to the substrate. Since enzymes are proteins, this site is composed of a unique combination of amino acid residues (side chains or R groups). Each amino acid rest can exist big or small; weakly acidic or basic; hydrophilic or hydrophobic; and positively-charged, negatively-charged, or neutral. The positions, sequences, structures, and properties of these residues create a very specific chemical surroundings inside the agile site. A specific chemical substrate matches this site like a jigsaw puzzle piece and makes the enzyme specific to its substrate.
Agile Sites and Environmental Weather condition
Environmental weather condition tin can touch an enzyme's active site and, therefore, the rate at which a chemical reaction can proceed. Increasing the ecology temperature generally increases reaction rates because the molecules are moving more quickly and are more likely to come into contact with each other.
Notwithstanding, increasing or decreasing the temperature outside of an optimal range can affect chemical bonds within the enzyme and change its shape. If the enzyme changes shape, the active site may no longer demark to the appropriate substrate and the rate of reaction will decrease. Dramatic changes to the temperature and pH will somewhen cause enzymes to denature.
Induced Fit and Enzyme Function
For many years, scientists thought that enzyme-substrate binding took place in a simple "lock-and-central" way. This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step. However, current inquiry supports a more than refined view chosen induced fit. As the enzyme and substrate come up together, their interaction causes a balmy shift in the enzyme's structure that confirms an platonic binding arrangement between the enzyme and the substrate. This dynamic bounden maximizes the enzyme'due south power to catalyze its reaction.

Induced Fit: According to the induced fit model, both enzyme and substrate undergo dynamic conformational changes upon binding. The enzyme contorts the substrate into its transition country, thereby increasing the rate of the reaction.
Enzyme-Substrate Complex
When an enzyme binds its substrate, it forms an enzyme-substrate complex. This complex lowers the activation energy of the reaction and promotes its rapid progression by providing certain ions or chemical groups that actually form covalent bonds with molecules every bit a necessary step of the reaction procedure. Enzymes also promote chemical reactions by bringing substrates together in an optimal orientation, lining upwards the atoms and bonds of one molecule with the atoms and bonds of the other molecule. This can contort the substrate molecules and facilitate bond-breaking. The active site of an enzyme likewise creates an ideal surround, such as a slightly acidic or non-polar surroundings, for the reaction to occur. The enzyme will always return to its original state at the completion of the reaction. I of the important backdrop of enzymes is that they remain ultimately unchanged by the reactions they catalyze. Afterward an enzyme is washed catalyzing a reaction, it releases its products (substrates).
Command of Metabolism Through Enzyme Regulation
Cells regulate their biochemical processes by inhibiting or activating enzymes.
Learning Objectives
Explicate the effect of an enzyme on chemical equilibrium
Key Takeaways
Key Points
- In competitive inhibition, an inhibitor molecule competes with a substrate by binding to the enzyme 's active site and then the substrate is blocked.
- In noncompetitive inhibition (also known equally allosteric inhibition), an inhibitor binds to an allosteric site; the substrate tin still bind to the enzyme, just the enzyme is no longer in optimal position to catalyze the reaction.
- Allosteric inhibitors induce a conformational change that changes the shape of the active site and reduces the affinity of the enzyme's active site for its substrate.
- Allosteric activators induce a conformational modify that changes the shape of the active site and increases the affinity of the enzyme'due south active site for its substrate.
- Feedback inhibition involves the use of a reaction product to regulate its own further production.
- Inorganic cofactors and organic coenzymes promote optimal enzyme orientation and function.
- Vitamins act equally coenzymes (or precursors to coenzymes) and are necessary for enzymes to role.
Key Terms
- coenzyme: An organic molecule that is necessary for an enzyme to function.
- allosteric site: A site other than the agile site on an enzyme.
- cofactor: An inorganic molecule that is necessary for an enzyme to function.
Control of Metabolism Through Enzyme Regulation
Cellular needs and atmospheric condition vary from cell to cell and modify within individual cells over time. For example, a stomach cell requires a different amount of free energy than a skin cell, fat storage cell, blood cell, or nervus jail cell. The same tummy prison cell may besides need more energy immediately later a meal and less energy between meals.
A cell'southward part is encapsulated past the chemic reactions it can behave out. Enzymes lower the activation energies of chemical reactions; in cells, they promote those reactions that are specific to the jail cell's office. Because enzymes ultimately decide which chemical reactions a cell tin carry out and the rate at which they tin proceed, they are fundamental to jail cell functionality.
Competitive and Noncompetitive Inhibition
The cell uses specific molecules to regulate enzymes in order to promote or inhibit certain chemical reactions. Sometimes it is necessary to inhibit an enzyme to reduce a reaction rate, and there is more than than one manner for this inhibition to occur. In competitive inhibition, an inhibitor molecule is similar enough to a substrate that information technology can bind to the enzyme's active site to stop it from bounden to the substrate. It "competes" with the substrate to bind to the enzyme.
In noncompetitive inhibition, an inhibitor molecule binds to the enzyme at a location other than the agile site (an allosteric site). The substrate can still bind to the enzyme, only the inhibitor changes the shape of the enzyme and so it is no longer in optimal position to catalyze the reaction.

Enzyme inhibition: Competitive and noncompetitive inhibition affect the rate of reaction differently. Competitive inhibitors affect the initial rate, merely practice not affect the maximal rate, whereas noncompetitive inhibitors affect the maximal rate.
Allosteric Inhibition and Activation
In noncompetitive allosteric inhibition, inhibitor molecules demark to an enzyme at the allosteric site. Their binding induces a conformational modify that reduces the affinity of the enzyme'due south agile site for its substrate. The binding of this allosteric inhibitor changes the conformation of the enzyme and its active site, and so the substrate is not able to demark. This prevents the enzyme from lowering the activation energy of the reaction, and the reaction rate is reduced.
However, allosteric inhibitors are not the merely molecules that bind to allosteric sites. Allosteric activators tin increase reaction rates. They bind to an allosteric site which induces a conformational change that increases the affinity of the enzyme'southward active site for its substrate. This increases the reaction charge per unit.

Allosteric inhibitors and activators: Allosteric inhibitors modify the active site of the enzyme and so that substrate binding is reduced or prevented. In contrast, allosteric activators change the agile site of the enzyme and so that the affinity for the substrate increases.
Cofactors and Coenzymes
Many enzymes only work if leap to non-protein helper molecules called cofactors and coenzymes. Binding to these molecules promotes optimal conformation and function for their corresponding enzymes. These molecules bind temporarily through ionic or hydrogen bonds or permanently through stronger covalent bonds.
Cofactors are inorganic ions such as iron (Fe2+) and magnesium (Mgii+). For case, DNA polymerase requires a zinc ion (Zn2+) to build DNA molecules. Coenzymes are organic helper molecules with a basic atomic structure made upwards of carbon and hydrogen. The most common coenzymes are dietary vitamins. Vitamin C is a coenzyme for multiple enzymes that take part in building collagen, an important component of connective tissue. Pyruvate dehydrogenase is a complex of several enzymes that requires i cofactor and v unlike organic coenzymes to catalyze its chemical reaction. The availability of various cofactors and coenzymes regulates enzyme role.
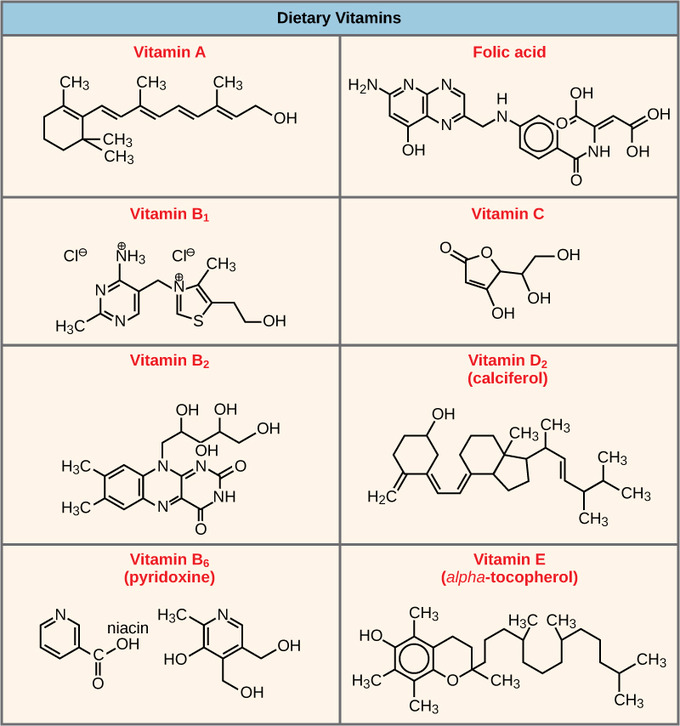
Vitamins: Vitamins are important coenzymes or precursors of coenzymes and are required for enzymes to function properly. Multivitamin capsules usually incorporate mixtures of all the vitamins at different percentages.
Enzyme Compartmentalization
In eukaryotic cells, molecules such as enzymes are usually compartmentalized into different organelles. This organization contributes to enzyme regulation because certain cellular processes are contained in divide organelles. For instance, the enzymes involved in the after stages of cellular respiration carry out reactions exclusively in the mitochondria. The enzymes involved in the digestion of cellular droppings and strange materials are located within lysosomes.
Feedback Inhibition in Metabolic Pathways
Feedback inhibition is when a reaction product is used to regulate its own further production. Cells have evolved to use feedback inhibition to regulate enzyme activity in metabolism, by using the products of the enzymatic reactions to inhibit further enzyme activity. Metabolic reactions, such as anabolic and catabolic processes, must go along according to the demands of the cell. In society to maintain chemical equilibrium and run across the needs of the cell, some metabolic products inhibit the enzymes in the chemical pathway while some reactants actuate them.

Feedback inhibition: Metabolic pathways are a series of reactions catalyzed by multiple enzymes. Feedback inhibition, where the cease production of the pathway inhibits an before step, is an important regulatory mechanism in cells.
The production of both amino acids and nucleotides is controlled through feedback inhibition. For an example of feedback inhibition, consider ATP. It is the product of the catabolic metabolism of sugar (cellular respiration), but it also acts as an allosteric regulator for the same enzymes that produced it. ATP is an unstable molecule that can spontaneously dissociate into ADP; if also much ATP were present, well-nigh of information technology would go to waste. This feedback inhibition prevents the production of boosted ATP if it is already abundant. However, while ATP is an inhibitor, ADP is an allosteric activator. When levels of ADP are loftier compared to ATP levels, ADP triggers the catabolism of sugar to produce more than ATP.
Source: https://courses.lumenlearning.com/boundless-biology/chapter/enzymes/
0 Response to "What Happens to an Enzyme After a Reaction"
Post a Comment